
A creative pharmaceutical packaging system manufacturer
Market Positioning
The company's products mainly serve pharmaceutical manufacturers, focusing on high-end pharmaceutical packaging needs in ophthalmology, dermatology and other sub-sectors, while also providing customized packaging solutions for in vitro diagnostic reagents. With its technological leadership and compliance, Zhonghui Technology has become a core supplier for many well-known domestic pharmaceutical companies and has gradually begun to expand into the international market.

Innovative Development
With the core development concept of "Quality builds life, innovation drives the future", Zhonghui Technology has built a complete quality management system and promoted technological upgrading through continuous R&D investment. The R&D team is led by senior technicians, focusing on new material development, process optimization and structural innovation, helping customers improve drug safety and usage experience.
Eye drop plug
CN201720580779.8
The use of a splash-proof quantitative drip eye drop cooling blow molding core rod plug can accurately control the dosage, which can better analyze the treatment process, treatment purpose and treatment effect. Without splashing and dripping, there will be no excessive and unnecessary waste of liquid medicine, and at the same time, patients can get a more comfortable medication process.
Precise Control
Circulation Cooling
Cooling blow molding mandrel
CN201821719542.4
The water is circulated in the rod core through the water inlet and outlet pipes to achieve the water cooling effect, thereby controlling the temperature of the bottle during thermoplasticization. The position relationship between the adjustment block and the connecting bolt and the extension distance of the connecting rod are adjusted to adjust the size of the blow molding gap and the speed of the airflow to meet the production requirements. In this way, the production parameters can be controlled with high precision to prevent the degradation of plastics during the processing process, and the degradation products affect the quality of the drug.
Circulation Cooling
Residue from dripping from the antibacterial bottle
liquid pre-treatment
CN201811492819.9
CN202410401726.X
The top sealing protection design of the liquid medicine outlet improves the overall sealing of the packaging system.
The 0.22 micron PTFE filter membrane and isolator are integrated into one to improve the system sealing integrity.
Gas-liquid channel integrated technology, pure dripping without residue.
Gas-liquid separation and residual liquid pre-evaporation and drying technology to avoid cross contamination of drug solutions.
No Preservatives

Oxygen Isolation
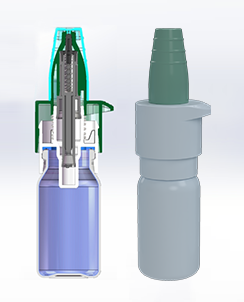
Quantitative drip, previent the invasion of oxygen and bacteria
CN202510222361.9
The front sealing valve design prevents the liquid from flowing back.
External return spring, no contact with the solution.
The multi-layer bag-in-bottle structure enables the pressure inside and outside the packaging system to be adaptively balanced without the need for air return.
Oxygen Isolation










